Class 9 Science Chapter 1 - Matter in Our Surrounding Notes
Nayaks Tutorials
Nayaks Tutorials
Class 9 Science Chapter 1, Matter in Our Surroundings, introduces the basic concept of matter, which makes up everything around us. This chapter explains how matter exists in different forms, how it behaves under various conditions, and the changes it undergoes. These notes are designed to help students grasp these fundamental concepts in a simple and clear way, making it easier to understand how the physical world works.
To enhance understanding and aid in exam preparation, students are encouraged to use comprehensive revision notes.
Everything in the universe is made up of material that occupies space and has mass, known as matter. Matter includes everything around us, from the air we breathe and the food we eat to stones, clouds, stars, plants, and animals. Even the smallest particle, like a drop of water or a grain of sand, is considered matter.
Solid: Particles are tightly packed in a fixed arrangement, giving solids a definite shape and volume. They are incompressible and have high density, like ice or metal.
Liquid: Particles are loosely packed, allowing them to move freely. Liquids have a definite volume but take the shape of their container. They are slightly compressible and flow easily, such as water or oil.
Gas: Particles are far apart and move independently, filling the entire space available. Gases have neither a definite shape nor volume, are highly compressible, and can expand, like oxygen or carbon dioxide.

Panch Tatva: Early Indian philosophers classified matter into five basic elements known as "Panch Tatva" – air, earth, fire, sky, and water.
Modern Classification: In contrast, modern scientists have evolved a classification system for matter based on its physical properties and chemical nature.
Chair, air, love, smell, hate, almonds, thought, cold, lemon water, smell of perfume.
Answer: Matter is anything that has mass and occupies space. Based on this definition, the following items from the list are considered matter:
• Chair
• Air
• Almonds
• Lemon water
The other items, such as love, smell, hate, thought, cold, and smell of perfume, are not considered matter because they do not have mass and do not occupy space. These are either emotions, sensations, or states, which do not qualify as matter.
Matter, which makes up everything around us, has three key characteristics that define its behavior:
Particles of matter are always in motion. This movement varies depending on the state of matter (solid, liquid, or gas) and is influenced by factors like temperature. For instance, particles in a gas move more freely and rapidly compared to those in solids or liquids.
There is always some amount of space between the particles of matter. This space determines the density of the material and how particles interact with each other. In solids, the particles are closely packed with minimal space between them, whereas in gases, the space between particles is much larger.
Particles of matter have a natural tendency to attract each other. The strength of this attraction varies depending on the type of matter. In solids, the particles are strongly attracted to each other, which gives solids their definite shape. In liquids, the attraction is weaker, allowing the particles to flow, and in gases, the attraction is very weak, allowing particles to move freely.
Understanding these characteristics is fundamental in explaining the behavior and properties of different states of matter.
Give reasons for the following observation:
The smell of hot sizzling food reaches you several metres away, but to get the smell from cold food you have to go close.
(i) When food is sizzling hot, it releases vapours of its contents.
(ii) Because on increasing the temperature of food, the kinetic energy of the particles increases, hence, they diffuse at a faster speed and reach us even at some distance.
(iii) However, no such vapours are released when the food is cold.
(iv) Therefore, we have to go close to it in order to get its smell.
A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Answer:This shows that in water which represents the liquid state of matter, there are sufficient inter particle spaces. That is why, a diver is able to cut through water. He might not do the same had these spaces been very small, e.g. in solid state.
Long Answer Questions (5 Marks)
What are the characteristics of the particles of matter?
(1) Particles of matter have space between them.
Answer:When we make tea, coffee or lemonade (nimbu paani), particles of one type of matter get into the spaces between particles of the other. This shows that there is enough space between particles of matter. Similarly, particles of sugar, salt, dettol, or potassium permanganate get evenly distributed in water.
(2) Particles of matter are continuously moving.
Answer:Particles of matter are continuously moving, that is, they possess what we call the kinetic energy. As the temperature rises, particles move faster. So, we can say that with increase in temperature, the kinetic energy of the particles also increases.
(3) Particles of matter attract each other.
Answer:Particles of matter have force acting between them. This force keeps the particles together. The strength of this force of attraction varies from one kind of matter to another.
1. Solid State:
• Tightly Packed Particles: In solids, particles are very closely packed together, giving solids their firm and fixed structure.
• Strong Particle Attraction: The force of attraction between particles in solids is very strong, keeping them in fixed positions.
• Definite Shape and Volume: Solids maintain a specific shape and volume because the particles do not move freely.
• High Density: Solids have a high density due to the close arrangement of their particles.
• Incompressibility: Solids cannot be easily compressed because the particles are already tightly packed.
• Non-Diffusive: Solids do not diffuse into other substances, as their particles are not free to move.
2. Liquid State:
• Loosely Packed Particles: Particles in liquids are less closely packed than in solids, allowing them to move around more freely.
• Moderate Particle Attraction: The attraction between particles in a liquid is weaker than in a solid, allowing for fluid movement.
• Indefinite Shape, Definite Volume: Liquids take the shape of their container but maintain a consistent volume.
• Lower Density than Solids: Liquids generally have a lower density compared to solids, enabling them to flow.
• Nearly Incompressible: Liquids are almost incompressible, though slightly more so than solids.
• Diffusion Ability: Liquids can diffuse into other liquids or gases, although more slowly than gases.
3. Gaseous State:
• Widely Spaced Particles: In gases, particles are far apart, giving gases their ability to expand and fill any container.
• Negligible Particle Attraction: The force of attraction between particles in gases is very weak, allowing them to move freely.
• No Definite Shape or Volume: Gases neither have a fixed shape nor a fixed volume; they expand to occupy the space available.
• Low Density: Gases have the lowest density among the three states of matter due to the large spaces between particles.
• Highly Compressible: Gases can be easily compressed because of the significant space between particles.
• Rapid Diffusion: Gases diffuse quickly into other gases because of their free-moving particles.
This overview summarizes the key differences in the arrangement and behavior of particles in solids, liquids, and gases, explaining why each state of matter has its own unique set of properties.
Tabulate the differences in the characteristics of states of matter. OR Comment upon the following: rigidity, compressibility, fluidity, filling a container, shape, kinetic energy and density.
Answer:| Property | Solids | Liquids | Gases |
| Mass | Definite mass | Definite mass | Definite mass |
| Shape | Definite shape | No definite shape | No definite shape |
| Volume | Definite Volume | Definite Volume | No definite Volume |
| Density | Highest | Lower than solids, higher than gases | Lowest |
| Rigidity | Highly rigid | Less rigid than solids | Not rigid |
| Fluidity | Do not flow | From higher level to low level | In all directions |
| Compressibility | Very low or cannot be compressed easily | Low | Very high |
| Free Surface | Infinite | One (upper surface) | No free surface |
| Diffusion | Cannot diffuse | Few diffuse immediately | Diffuse rapidly |
| Thermal Expansion | Low and Linear | Higher than solids | Highest |
| Packing of particles | Tightly packed | Loosely packed | Very loosely packed |
| Spaces between particles | Very less | Greater space as compared to solids | Greatest |
| Forces of Attraction | Very strong | Less than solids | Least |
Give reasons:
(a) A gas completely fills the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
Answer :(a) A gas completely fills the vessel in which it is kept because due to high kinetic energy and negligible forces of attraction, the molecules of a gas are moving with high speed in all directions.
(b) The kinetic energy of the particles in the gaseous state is maximum. Particles move about randomly at high speed. Due to random movement, the particles hit each other and also the walls of the container.
The pressure exerted by the gas is because of the force exerted by the gas particles per unit area on the walls of the container. (c) A wooden table should be called a solid because (i) it is rigid and cannot be compressed easily (ii) it has a definite volume and definite shape.
(d) It is because the intermolecular forces in wood (solid) are the strongest and negligible in air (gas).
• Physical states of matter can be changed from one form to another in two primary ways:
1. By changing the temperature
2. By changing the pressure
• Diffusion: The process where particles of two different types of matter mix on their own is known as diffusion.
• Latent Heat of Fusion: This is the amount of heat energy required to convert 1 kg of a solid into a liquid at atmospheric pressure and its melting point.
• Latent Heat of Vaporization: This is the heat energy needed to convert 1 kg of a liquid into gas at atmospheric pressure and its boiling point.
• Sublimation: Sublimation is the process in which a solid changes directly into a gas without passing through the liquid state. Examples include solid carbon dioxide (dry ice), ammonium chloride, and camphor.
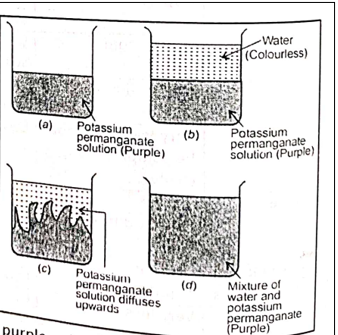
Explain the diffusion of potassium permanganate solution in water.
Ans: Potassium permanganate is a purple coloured solid substance which dissolves in water to form a purple coloured solution. The purple coloured potassium permanganate solution is kept in a beaker [Fig. (a)]. Tilt the beaker containing potassium permanganate solution slightly and add some clear water to it carefully along the sides of the beaker without disturbing the purple coloured solution. In this way, we will get two distinct layers of liquids in the beaker, the lower layer of purple coloured potassium permanganate solution and the upper layer of colourless water [Fig. (b)]. Soon we will see the purple colour of potassium permanganate solution spreading upwards into clear water and clear water moving downwards into purple solution [Fig. (c)]. After some time, the whole this stage, the potassium permanganate solution and water mix with each other completely to form a uniformly coloured purple solution. The spreading of purple colour into whole water is due to the diffusion of potassium permanganate solution and clear water into each other.
• Increase in Surface Area: Evaporation is a surface phenomenon. An increase in surface area speeds up evaporation, such as spreading clothes out to dry.
• Increase in Temperature: Higher temperatures provide more kinetic energy to particles, enabling them to evaporate more quickly.
• Decrease in Humidity: Lower humidity increases evaporation since less water vapor in the air allows more liquid to turn into vapor.
• Increase in Wind Speed: Higher wind speeds remove water vapor from the air near the liquid surface, allowing more liquid to evaporate.
• Evaporation: The process by which a liquid changes into vapor at temperatures below its boiling point. Evaporation is a surface phenomenon.
• Boiling Point: The temperature at which a liquid starts boiling at atmospheric pressure. Boiling is a bulk phenomenon, meaning it affects the entire liquid, not just the surface.
These notes cover essential aspects of how matter changes states, the energy involved in these processes, and how external factors can influence the rate at which these changes occur.
Convert the following temperatures to celsius scale: (a) 300 K (b) 573 K.
Answer: Temperature on Kelvin scale = Temperature on Celsius scale + 273
(a) 300 = Temperature on Celsius scale + 273
or Temperature of Celsius scale = 300 - 273 = 27°C
(b) 573 = Temperature on Celsius scale + 273
or Temperature on Celsius scale = 573 - 273 = 300°C
Convert the following temperature to Kelvin scale: (a) 25°C (b) 373°C
Answer: (a) K = C + 273
∴ K = 25 + 273
∴ K = 298 K.
(b) K = C + 273
∴ K = 373 + 273
∴ K = 646 K.
Answer: (i) The cooling in a desert room cooler is caused by the evaporation of water.
(ii) A desert cooler cools better on a hot and dry day because the higher temperature on a hot day increases the rate of evaporation of water and the dryness of air (low humidity of air) also increases the rate of evaporation of water.
(iii) Due to the increased rate of evaporation of water, a desert cooler cools better on a hot and dry day.
We sweat more in summer. As the sweat evaporates it takes energy from our body surface and keeps our body cool. Cotton can absorb the sweat easily and exposes it to the atmosphere causing evaporation to take place easily. This, in turn, keeps us cool on summer days.
There are water vapours present in the air. When they come in contact with the walls of the glass that has ice-cold water in it they condense. As a result, their state changes from the gaseous state to liquid state thus forming tiny water droplets on the walls of the glass.
There are many small pores in an earthen pot. Through these pores, the water kept inside the pot evaporates. It takes the latent heat required for vaporization from the earthen pot and the remaining water in the earthen pot. Hence water loses heat making the water inside the pot cool.
Answer: (a) Naphthalene balls disappear without leaving any solid substance because they sublime. They change directly from solid to gas without changing into a liquid state.
(b) We can get the smell of perfume sitting several metres away. The particles of perfume mix with the particles of air around us and spread out. Due to this spreading of particles, we are able to get the smell even sitting several metres away.
Answer: Cooling takes place when heat is removed from a system. In case of ice at 272 K, it will take latent heat from the medium to convert itself into water at 273 K and then into water at a higher temperature. Thus, in case of ice at 273 K there will be a change in physical state, whereas in case of water at 273 K there will be no change in state. Hence, lesser energy will be taken from the medium.
In conclusion, the Class 9 Science Chapter 1 notes on "Matter in Our Surroundings" provide a thorough understanding of the essential concepts related to the physical properties and behavior of matter. These notes cover how matter changes states, the influence of temperature and pressure, and the distinct characteristics of solids, liquids, and gases. They also delve into key processes like diffusion, evaporation, and sublimation, along with the factors that affect the rate of evaporation. By studying these well-structured "Matter in Our Surroundings" Class 9 notes and joining CBSE coaching classes for 9th and 10th standard at Nayak's Tutorials, students can enhance their grasp of these crucial concepts. This strong foundation will not only help in excelling in exams but also prepare them for more advanced studies in the field of physical sciences.